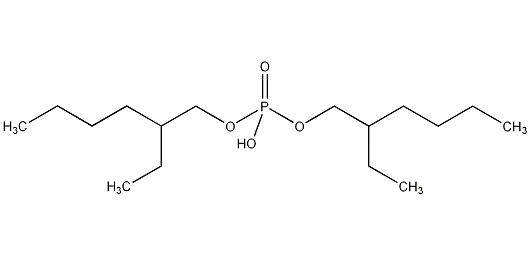
Is P204 Toxic or Hazardous?
Di(2-ethylhexyl) Phosphate (P204) is considered a moderately toxic substance. It can cause irritation to the skin, eyes, and respiratory system upon direct contact or inhalation. It is essential to handle P204 with appropriate safety precautions, including the use of protective equipment and adherence to recommended handling procedures.
Are There Any Environmental Concerns Associated with P204?
Di(2-ethylhexyl) Phosphate (P204) has low aquatic toxicity and is not persistent in the environment. However, like other organophosphates, it should be handled and disposed of responsibly to prevent contamination of water bodies and ecosystems.